In Cellular Respiration Oxygen Gain or Lose Electrons
Inside an active mitochondrion most electrons follow which. Supplements can play a role - but the basis of weight gain is nutrition - not pills and potions.
Introduction To Cellular Respiration And Redox Article Khan Academy
While RPGs are a diverse genre they are For gain media operating on allowed transitions rather than forbidden transitions such as laser dyes or semiconductors the cross sections are much higher.

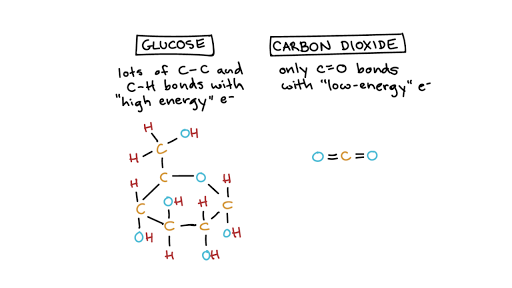
. The energy required to gain or lose 4 electrons is immense due to the attractive force between protons and electrons in the nucleus. To complete an octet. Valency is defined as the total number of electrons an atom can lose gain or share at the time of bond formation to get a stable electronic configuration ie.
Provide your weight exerciseactivity level and your personal fitness goals. Get customized meal plans healthy macro-friendly recipes tips access to a nutrition coach and more. Oxidation is the loss of electrons from one substance and reduction is the gain of electrons to another substance.
Let us see the four steps involved in brief before we move into the details of what is the cellular respiration equation. _____is the reducing agent in this reaction and. This oxygen gas is identical to the oxygen gas given off in photosynthesis.
Metabolism Questions and Answers. Hexalto Mar 14 2020. Many reactions in organic chemistry are redox reactions due to changes in oxidation states but without distinct electron transfer.
A to donate high energy electrons to the electron transport chain B to serve as an acceptor for released carbon forming C02 C to serve as an acceptor for electrons and protons forming water D to donate high energy electrons to NAD forming NADH. Get help with your Metabolism homework. The valency of an atom can be variable in different compounds or chemical reactions due to the different bonding circumstances.
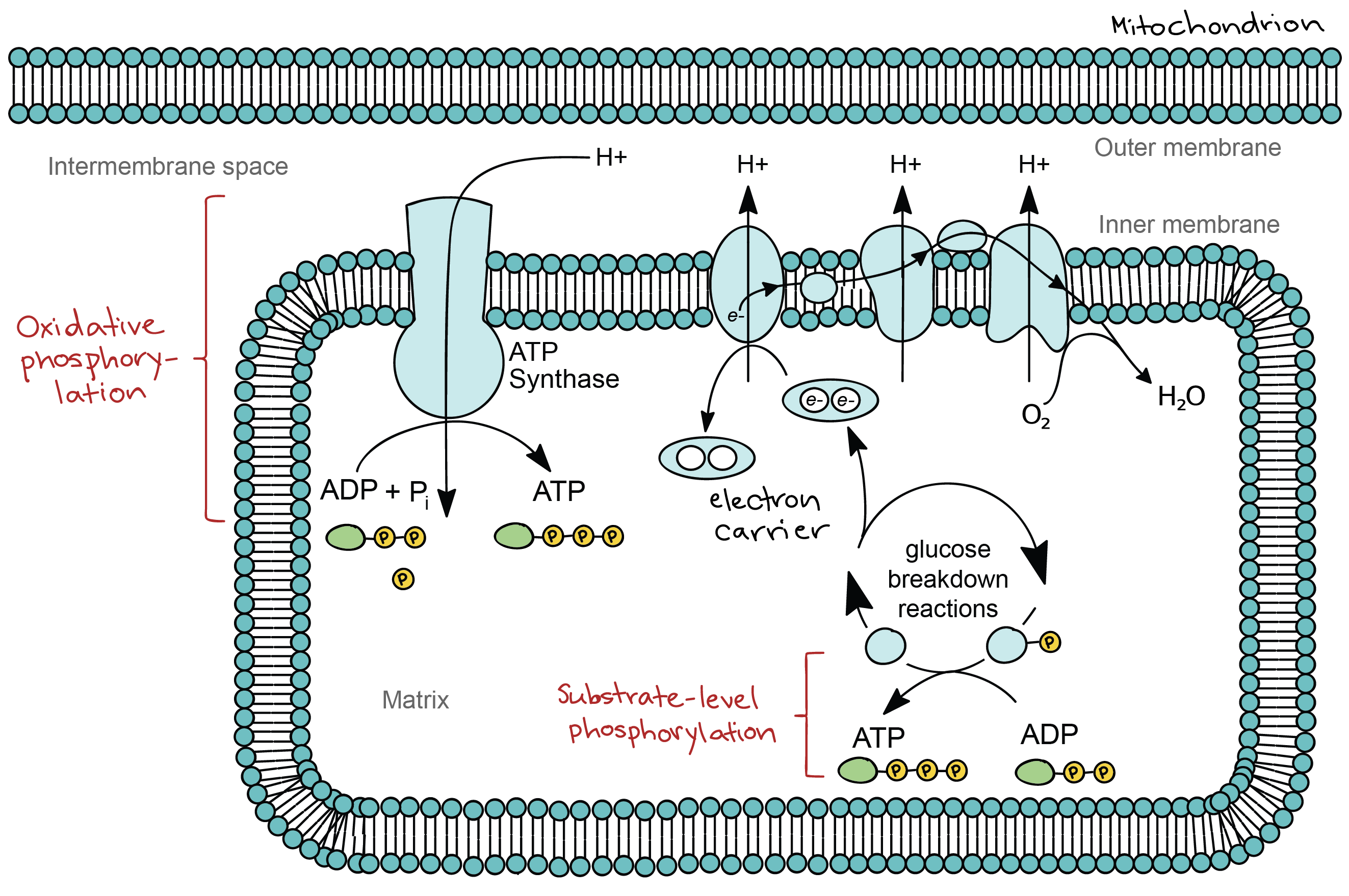
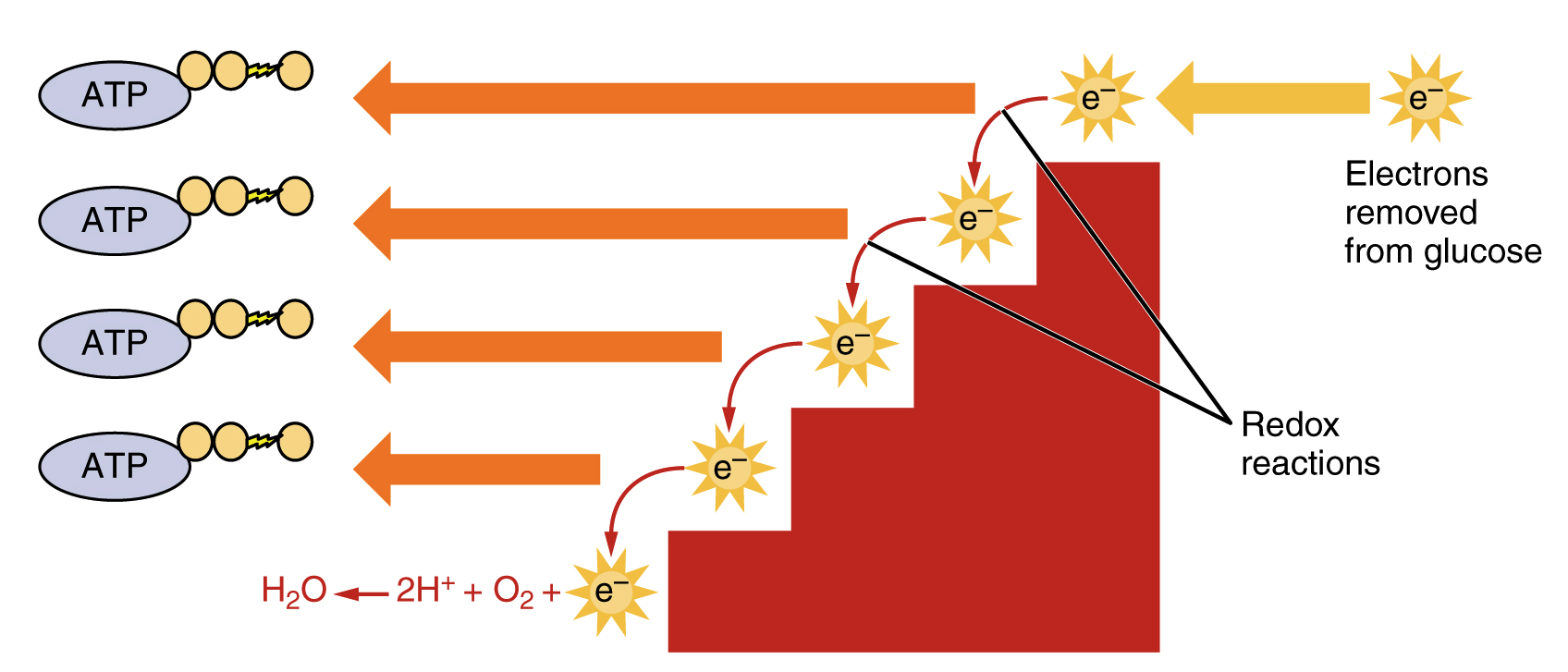
Most of the time valency varieschanges due to change in. The overall mechanism of cellular respiration involves four subdivisions. The electron transport chain is composed of four large multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers.
Oxygen is readily found in the earths atmosphere as well as in the overall makeup of the universe. For example during the combustion of wood with molecular oxygen the oxidation state of carbon. The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism.
An oxygen atom consists of 6 valence electrons in its outermost layer. It shares two electrons with another oxygen atom through a non-polar covalent bond to become a stable diatomic molecule. So feel free to try different rp.
Perhaps the best known example of a redox reaction is aerobic heterotrophic respiration with molecular oxygen O 2 acting as an electron acceptor during the oxidation of. Glycolysis in which glucose molecules are broken down to form pyruvic acid molecules. Similarly oxygen has 2 electrons in the K shell and 6 electrons in the L shell.
Also in the process of cellular respiration oxygen gas is required to serve as an acceptor of electrons. It might not be obvious at first but if you want more flexibility focus more on your breath than on your muscles. There are 6 oxygen 6 carbon dioxide and 6 water molecules.
Weight Gain RP - TheThis amino is mainly for weight gain roleplay. Weight Gain Roleplay A little book of weight gain roleplaying All plots are written. The Krebs cycle in which pyruvic.
Oxygen O 2. A solution with a higher ORP will have a tendency to gain electrons ie oxidize them and a solution with a lower ORP will have a tendency to lose electrons to new species ie reduce them. T time in.
Xe Y X Ye Which component is oxidized and which component is reduced. Access the answers to hundreds of Metabolism questions that are explained in a. 40-minute cardio before breakfast.
Fermentation is the partial degradation of sugars or other organic fuel without oxygen while cellular respiration uses oxygen. Reduction is the gain of electrons or a decrease in the oxidation state of an atom an ion or of certain atoms in a molecule a reduction in oxidation state. What is the primary role of oxygen in cellular respiration.
Photosynthesis is the process used by plants algae and certain bacteria to turn sunlight carbon dioxide and water into food sugars and oxygen. There are four distinct processes that divide the total cellular respiration process. However the middle ground option is to share its electrons.
It only needs 2 electrons to be stable and attain an electronic configuration similar to neon. Cellular respiration is different from photosynthesis and is usually an aerobic reaction that occurs in the presence of oxygen. Vilsbøll T Christensen M Junker AE Knop FK Gluud LL.
If you feel that you need someone to talk to our trained crisis workers are available Med Sci Sports Exerc.

8 1 Cell Respiration A Biology
Introduction To Cellular Respiration And Redox Article Khan Academy
Introduction To Cellular Respiration And Redox Article Khan Academy
No comments for "In Cellular Respiration Oxygen Gain or Lose Electrons"
Post a Comment